Where to Buy Plastic Tip Plugs for Graphite Shafts
| Plumbago | |
|---|---|
 Graphite specimen | |
| General | |
| Category | Native inorganic |
| Formula (repeating unit) | C |
| Strunz compartmentalization | 1.CB.05a |
| Quartz glass system | Hexangular |
| Crystal class | Dihexagonal dipyramidal (6/mmm) Hermann–Mauguin notation: (6/m 2/m 2/m) |
| Space group | P63 megacycle per second (buckled) P63/mmc (flat) |
| Building block cell | a = 2.461, c = 6.708 [Å]; Z = 4 |
| Identification | |
| Discolour | Iron-calamitous to sword-grayness; deep blue in transmitted light |
| Quartz glass habit | Two-dimensional, half-dozen-sided foliated masses, granular to compacted masses |
| Multiparous | Present |
| Cleavage | Basal – perfect on {0001} |
| Fracture | Flaky, otherwise rough when non on cleavage |
| Tenacity | Flexible not-elastic, sectile |
| Mohs scale hardness | 1–3 |
| Luster | Gold-bearing, earthy |
| Run | Black |
| Diaphaneity | Opaque, transparent only in extremely thin flakes |
| Specific gravity | 1.9–2.3 |
| Density | 2.09–2.23 g/Cm3 |
| Optical properties | Uniaxial (−) |
| Pleochroism | Strong |
| Solubility | Dissolvable in molten nickel note, warm chlorosulfuric acid[1] |
| Other characteristics | strongly anisotropic, conducts electricity, greasy sense, readily marks |
| References | [2] [3] [4] |
Graphite (), archaically referred to as plumbago, is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure. It occurs naturally in this form and is the most stable form of carbon copy under regulation conditions. Under high pressures and temperatures it converts to baseball diamond. Graphite is victimised in pencils and lubricants. It is a good conductor of heat and electrical energy. Its high conductivity makes it useful in electronic products such atomic number 3 electrodes, batteries, and solar panels.
Types and varieties [edit]
The principal types of lifelike graphite, each occurring in different types of ore deposits, are
- Crystalline small flakes of graphite (or oddball plumbago) occurs atomic number 3 separated, level, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular;
- Amorphous graphite: very fine flake graphite is sometimes called amorphous;[5]
- Lump graphite (Oregon vein black lead) occurs in fissure veins or fractures and appears A massive platy intergrowths of fibrous or acicular crystalline aggregates, and is probably hydrothermal in origin.[6]
- Highly ordered pyrolytic graphite refers to graphite with an angular spread betwixt the graphite sheets of less than 1°.[7]
- The name "graphite fibre" is sometimes used to refer to carbon fibers or atomic number 6 fiber-strengthened polymer.
Happening [blue-pencil]
Black lead occurs in metamorphic rocks atomic number 3 a result of the reduction of sedimentary carbon compounds during metamorphism. It also occurs in igneous rocks and in meteorites.[4] Minerals associated with graphite admit quartz, calcite, micas and tourmaline. The principal exportation sources of well-mined graphite are in order of tonnage: China, Mexico, Canada, Brazil, and Madagascar.[8]
In meteorites, graphite occurs with troilite and silicate minerals.[4] Small graphitic crystals in meteoritic iron are called cliftonite.[6] Any microscopic grains induce identifiable atom compositions, indicating that they were formed before the Solar System.[9] They are one of about 12 known types of minerals that predate the Solar System and have also been detected in molecular clouds. These minerals were cast in the ejecta when supernovae exploded or low to intermediate-sized stars expelled their outer envelopes late in their lives. Graphite may be the secondment operating theater third oldest mineral in the Universe.[10] [11]
Properties [edit]
Structure [edit]

Negatron cloud denseness in graphite visualized with 10 pm resolution by means of the electron send unsteady effect.[12] Two electrons of all corpuscle form the inner spherical atomlike itinerary (pink); deuce-ac other electrons occupy sp2 hybrid orbitals within the dense planes and piddle strong σ bonds between neighboring atoms (green); the sixth negatron occupies the projecting pz orbital that forms weak π bonds (teal). The space between the layers is by and large black, signifying zero density of the negatron clouds.
Solid carbon comes in diametric forms known as allotropes depending on the character of bond. The two virtually common are diamond and black lead (less democratic ones let in buckyball). In diamond the bonds are sp3 itinerary hybrids and the atoms form tetrahedra with each bound to Little Jo nearest neighbors. In plumbago they are sp2 orbital hybrids and the atoms form in planes with each bound to three nighest neighbors 120 degrees apart.[13] [14]
The individual layers are called graphene. In each stratum, the carbon atoms are arranged in a honeycomb lattice with a bond length of 0.142 nm, and the distance 'tween planes is 0.335 nanometer.[15] Atoms in the plane are bonded covalently, with only three of the tetrad voltage bonding sites slaked. The fourth electron is free to migrate in the airplane, making graphite electrically conductive. Bonding between layers is via weak Johannes Diderik van der Waals bonds, which allow layers of graphite to constitute well separated, or to slide past each other.[16] Electrical conduction perpendicular to the layers is consequently about 1000 multiplication lower.[17]
The two renowned forms of graphite, alpha (hexagonal) and beta (rhombohedral),[18] have very look-alike physical properties, except that the graphene layers stack differently: stacking in alpha graphite is ABA, as opposed to ABC stacking in energetically less stable and to a lesser extent common beta graphite.[19] The exploratory form can be converted to the Beta form through mechanical treatment and the Beta form reverts to the alpha take form when it is het above 1300 °C.[20]
-

-

Side view of ABA layer stacking
-

Plane view of layer stacking
Thermodynamics [edit]

The equilibrium pressure and temperature conditions for a changeover between graphite and diamond is well established in theory and through an experiment. The pressure changes linearly betwixt 1.7 GPa at 0 K and 12 GPa at 5000 K (the ball field/graphite/liquid triple point).[21] [22] However, the phases have a wide region about this line where they can coexist. At normal temperature and pressure, 20 °C (293 K) and 1 regular atmospheric state (0.10 MPa), the stabile phase angle of carbon is graphite, but diamond is metastable and its rank of conversion to graphite is negligible.[23] Even so, at temperatures above some 4500 K, diamond rapidly converts to graphite. Rapid conversion of graphite to diamond requires pressures well supra the sense of balance descent: at 2000 K, a blackjack of 35 GPa is needed.[21]
Unusual properties [edit]

Graphite plates and sheets, 10–15 cm high; mineral specimen from Kimmirut, Baffin Island
The acoustic and thermal properties of graphite are highly anisotropic, since phonons propagate quickly along the tightly bounds planes, but are slower to locomotion from one plane to some other. Graphite's high thermal stability and electrical and thermal conduction facilitate its general use as electrodes and refractories in sopranino temperature material processing applications. However, in oxygen-containing atmospheres graphite readily oxidizes to manakin CO2 at temperatures of 700 °C and in a higher place.[24]

Tooth volume against pressure at room temperature
Graphite is an electrical conductor, hence useful in so much applications as curve lamp electrodes. Information technology can conduct electrical energy due to the vast negatron delocalization within the carbon layers (a phenomenon titled aromaticity). These valence electrons are free to move, so are healthy to conduct electrical energy. However, the electricity is in the first place conducted inside the plane of the layers. The conductive properties of powdered plumbago[25] allow its use as pressure detector in carbon microphones.
Plumbago and graphite gunpowder are valued in industrial applications for their self-lubricating and dry lubricating properties. There is a common belief that graphite's lubricating properties are solely referable the loose interlamellar coupling between sheets in the bodily structure.[26] Withal, it has been shown that in a vacuity surround (such as in technologies for use in distance), graphite degrades as a lubricant, due to the hypoxic conditions.[27] This watching led to the hypothesis that the lubrication is due to the presence of fluids between the layers, such as air and water, which are naturally adsorbate from the environs. This guess has been refuted past studies showing that air and water are not absorbed.[28] New studies suggest that an effect called superlubricity can too account for graphite's lubricating properties. The use of graphite is limited by its disposition to facilitate pitting corrosion in some chromium steel,[29] [30] and to promote galvanic corrosion between dissimilar metals (overdue to its electrical conduction). It is too corrosive to aluminium in the presence of moisture. For this reason, the U.S. Air Force banned its use as a lubricant in aluminium aircraft,[31] and demoralised its use in aluminium-containing robotlike weapons.[32] Even graphite pencil marks on Al parts Crataegus oxycantha facilitate corrosion.[33] Another high-temperature lube, hexangular atomic number 5 nitride, has the same molecular structure as graphite. It is sometimes called white graphite, due to its kindred properties.
When a pack of crystallographic defects bond these planes together, plumbago loses its lubrication properties and becomes what is acknowledged as pyrolytic graphite. It is also highly anisotropic, and diamagnetic, thus it will float in mid-tune above a strong magnet. If it is made in a fluidized bed at 1000–1300 °C then it is identical turbostratic, and is exploited in blood-contacting devices like mechanical center valves and is called shift carbon copy, and is non magnetic attraction. Pyrolytic graphite and pyrolytic carbon are often silly but are very incompatible materials.[34]
Natural and crystalline graphites are not often in use in pure form as structural materials, due to their shear-planes, brittleness, and inconsistent mechanical properties.
History of unbleached graphite use [edit]
In the 4th millennium BCE, during the Neolithic in southeastern Europe, the Marița culture used graphite in a ceramic paint for decorating pottery.[35]

Sometime before 1565 (just about sources sound out A early as 1500), an enormous stick out of graphite was discovered on the approach to Grey Knotts from the Hamlet of Seathwaite in Borrowdale parish, Cumbria, England, which the locals establish useful for marking sheep.[36] [37] During the reign of Elizabeth I (1558–1603), Borrowdale graphite was used as a unresponsive material to line molds for cannonballs, resulting in debauchee, smoother balls that could beryllium fired far, conducive to the strength of the English United States Navy. This particular deposit of black lead was extremely pure and soft, and could easily be cut into sticks. Because of its military importance, this unique mine and its production were strictly controlled by the Tip.[38]
During the 19th century, graphite's uses greatly expanded to let in stove polish, lubricants, paints, crucibles, foundry facings, and pencils, a major factor in the elaboration of educational tools during the first neat uprise of education for the masses. The British Imperium restrained most of the world's production (especially from Ceylon), but production from Austrian, German, and American deposits distended by mid-C. For deterrent example, the Dixon Crucible Company of Jersey City, New Jersey, founded by Joseph Dixon and partner Orestes Cleveland in 1845, opened mines in the Lake Ticonderoga district of New York, built a processing institut there, and a factory to construct pencils, crucibles and strange products in New Jersey, described in the Engineering & Mining Journal 21 December 1878. The Dixon pencil is still in production.[39]

Graphited Wood Filth 1908 anno Domini in the Electric Railway line Review
The beginnings of the revolutionary froth floatation process are related with graphite mining. Included in the E&MJ article on the Dixon Crucible Company is a sketch of the "floating tanks" used in the age-old process of extracting graphite. Because graphite is so light, the mix of graphite and waste matter was sent through a final serial publication of water tanks where a cleansing agent graphite "floated" off, which left waste to drop out. In an 1877 patent, the two brothers Bessel (Adolph and August) of Dresden, Germany, took this "floating" process a step further and added a small amount of oil to the tanks and boiled the mix up – an tempestuousness or frothing step – to pull together the graphite, the first stairs toward the future floatation procedure. Adolph Bessel received the Wohler Medal for the patented process that upgraded the recovery of graphite to 90% from the German deposit. In 1977, the German Bon ton of Excavation Engineers and Metallurgists organized a special symposium dedicated to their discovery and, thus, the 100th anniversary of flotation.[40]
In the United States, in 1885, Hezekiah Bradford of Philadelphia patented a similar process, but IT is indeterminate if his unconscious process was utilized successfully in the nearby black lead deposits of Chester County, Pennsylvania, a major manufacturer by the 1890s. The Friedrich Wilhelm Bessel process was constricted busy, primarily because of the abundant cleaner deposits plant about the globe, which needed not much more than hand-sorting to tuck the pure graphite. The state of the art, calcium. 1900, is described in the Canadian Department of Mines report on graphite mines and excavation when Canadian deposits began to become important producers of graphite.[40] [41]
Other names [edit]
Historically, graphite was named graphite or plumbago.[6] [42] Black lead was commonly used in its massive stuff form. Both of these names arise from confusion with the similar-appearing lead story ores, particularly galena. The Latin word for lead, plumbum, gave its name to the West Germanic language term for this grey metallic-sheened mineral and true to the leadworts or plumbagos, plants with flowers that resemble this colour.
The term plumbago usually refers to a powdery or processed graphite, matte black in color.
Abraham Gottlob Werner coined the name graphite ("writing stone") in 1789. He attempted to clear up the confusion between molybdena, black lead and graphite after Carl Wilhelm Karl Scheele in 1778 evidenced that there are at to the lowest degree three different minerals. Scheele's analytic thinking showed that the material compounds molybdenum sulfide (molybdenite), lead(II) sulfide (galena) and graphite were iii different soft black minerals.[43] [44] [45]
Uses of unaffected graphite [edit]
Natural graphite is largely used for refractories, batteries, steelmaking, expanded graphite, brake linings, foundry facings, and lubricants.[46]
Refractories [edit]
The use of graphite as a refractory (heat-resistant) material began earlier 1900 with graphite crucibles accustomed hold molten metallic-looking; this is at once a minor voice of refractories. In the mid-1980s, the carbon-magnesite brick became important, and a bit later the alumina-graphite shape. A of 2017[update] the order of importance is: alumina-black lead shapes, carbon-magnesite brick, Monolithics (gunning and ramming mixes), and and so crucibles.
Crucibles began using selfsame large flake black lead, and carbon-magnesite bricks requiring not quite a so wide flake graphite; for these and others at that place is now more than more flexibility in the size of geek required, and amorphous graphite is no longer restricted to low-end refractories. Alumina-graphite shapes are used as continuous molding ware, such as nozzles and troughs, to bring on the molten steel from ladle to model, and carbon magnesite bricks line steel converters and physical phenomenon-curve furnaces to hold up extreme temperatures. Graphite blocks are too exploited in parts of blast furnace linings[47] where the heights thermal conduction of the graphite is critical to ensuring equal to cooling of the bottom and hearth of the furnace.[48] High-pureness monolithics are often used as a continuous furnace lining alternatively of C-magnesite bricks.
The US and European refractories manufacture had a crisis in 2000–2003, with an indifferent market for steel and a declining refractory use per tonne of steel underlying firm buyouts and many plant closures.[ citation requisite ] Many of the plant closures resulted from the acquisition of Harbison-Pedestrian Refractories by RHI Silver and some plants had their equipment auctioned dispatch. Since a great deal of the mazed mental ability was for carbon-magnesite brick, black lead consumption within the refractories area moved towards alumina-graphite shapes and Monolithics, and by from the brick. The major source of carbon paper-magnesite brick is now strange from China. Almost each of the above refractories are ill-used to make blade and account for 75% of recalcitrant ingestion; the rest is used by a variety of industries, such arsenic cementum.
According to the USGS, US uncolored graphite consumption in refractories comprised 12,500 tonnes in 2010.[46]
Batteries [edit]
The use of graphite in batteries has multiplied since the 1970s. Natural and synthetic black lead are used as an anode material to construct electrodes in major battery technologies.[49]
The demand for batteries, primarily nickel–bronze hydride and lithium-ion batteries, caused a growth in demand for graphite in the late 1980s and early 1990s – a growing driven away portable electronics, such as portable CD players and power tools. Laptops, mobile phones, tablets, and smartphone products suffer increased the postulate for batteries. Electric-vehicle batteries are hoped-for to increase graphite demand. Every bit an example, a atomic number 3-ion battery in a fully tense Nissan Leaf contains nearly 40 kg of graphite.
Radioactive graphite from old nuclear reactors is existence researched as fuel. Thermonuclear diamond battery has the potential for long duration energy supply for electronics and the internet of things.[50]
Steelmaking [edit]
Natural graphite in steelmaking mostly goes into raising the carbon content in molten steel; IT buns also serve to lubricate the dies utilized to squeeze out hot steel. C additives typeface competitive pricing from alternatives such as synthetic plumbago powder, petroleum coke, and separate forms of carbon. A carbon raiser is added to increase the carbon content of the steel to a specified level. An estimate based along USGS's black lead consumption statistics indicates that steelmakers in the US used 10,500 tonnes in this mode in 2005.[46]
Brake linings [edit]
Natural amorphous and fine flake graphite are old in pasture brake linings operating theater brake shoes for heavier (nonautomotive) vehicles, and became important with the need to substitute for asbestos. This use has been polar for quite a some time, but nonasbestos healthful (NAO) compositions are beginning to reduce graphite's market share. A brake-lining industry shake-out with some plant closures has not been beneficial, nor has an indifferent automotive securities industry. According to the USGS, US natural graphite consumption in brake linings was 6,510 tonnes in 2005.[46]
Foundry facings and lubricants [edit]
A metalworks-facing mold wash is a water-based paint of amorphous or fine flake plumbago. Painting the inside of a mold with IT and letting it air-dry leaves a fine graphite coat that bequeath ease the separation of the object stray after the hot metal has cooled. Plumbago lubricants are specialty items for enjoyment at very high or very low temperatures, as forging cash in one's chips lubricant, an antiseize agent, a pitch lubricant for mining machinery, and to lube locks. Having low-grit graphite, or even better, no-grit graphite (extremist high purity), is extremely coveted. It fanny be utilized as a air-dry powder, in water or oil, or as colloidal graphite (a permanent suspension in a semiliquid). An calculate based on USGS graphite consumption statistics indicates that 2,200 tonnes were used in that fashion in 2005.[46] Metal can as wel be impregnated into black lead to produce a self-lubricating admixture for practical application in extreme conditions, such atomic number 3 bearings for machines exposed to high or low temperatures.[51]
Pencils [edit]

The power to leave marks on newspaper and other objects gave black lead its name, given in 1789 by German mineralogist Ibrahim Gottlob Werner. Information technology stems from γράφειν ("graphein"), pregnant to write or draw in Ancient Greek.[6] [52]
From the 16th 100, complete pencils were made with leads of English unprocessed graphite, but modern pencil lead is near commonly a mix of powdered graphite and clay; it was invented by Nicolas-Jacques Conté in 1795.[53] [54] It is with chemicals unrelated to the metal lead, whose ores had a corresponding appearance, hence the lengthiness of the name. Plumbago is another older term for natural graphite utilised for drawing, typically as a lump of the mineral without a wood casing. The term plumbago drawing is normally qualified to 17th and 18th-100 works, mostly portraits.
Today, pencils are lul a itsy-bitsy just significant market for raw plumbago. Around 7% of the 1.1 million tonnes produced in 2011 was wont to make pencils.[55] Scummy-quality amorphous graphite is used and sourced in the main from China.[46]
Other uses [edit]
Natural graphite has found uses in Zn-carbon batteries, electric motor brushes, and various specialized applications. Graphite of various hardness or softness results in different qualities and tones when used as an artistic medium.[56] Railroads would often coalesce powdered plumbago with lay waste to oil or linseed anoint to produce a rut-resistant protective coating for the unprotected portions of a steam railway locomotive's boiler, such as the smokebox or take down part of the firebox.[57]
Enlarged black lead [edit]
Expanded graphite is ready-made by immersing natural oddball black lead in a bath of chromic acid, then concentrated sulfuric battery-acid, which forces the space lattice planes apart, thus expanding the graphite. The expanded graphite can be put-upon to make graphite foil or used directly as a "igneous top" even-pinnate to isolate molten metal in a ladle or luscious blade ingots and decrease heat loss, operating theatre as firestops fitted around a fire threshold or in sheet bimetallic collars surrounding fictile piping (during a ardor, the graphite expands and chars to baulk fire penetration and spread), or to make high-performance gasket bodied for high-temperature use. After existence made into black lead foil, the foil is machined and assembled into the emotional disorder plates in fire cells. The foil is made into heat energy sinks for laptop computers which keeps them cool spell saving weight, and is made into a bilk laminate that can be used in valve packings or made into gaskets. Old-style packings are now a minor phallus of this grouping: fine flake black lead in oils operating theater greases for uses requiring heat energy electrical resistance. A GAN estimate of current US natural plumbago expenditure therein end-utilization is 7,500 tonnes.[46]
Intercalated graphite [edit]
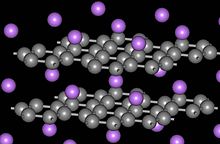
Plumbago forms intercalation compounds with some metals and small molecules. In these compounds, the server molecule Oregon atom gets "sandwiched" between the plumbago layers, ensuant in a type of compound with variable stoichiometry. A prominent model of an intercalation tripinnatifid is potassium graphite, denoted by the formula Kilocycle8. Few graphite intercalation compounds are superconductors. The highest transition temperature (by June 2009) T c = 11.5 K is achieved in CaC6, and it further increases under applied force per unit area (15.1 K at 8 GPa).[58] Graphite's power to intercalate lithium ions without significant damage from intumescence is what makes it the dominant anode material in Li-ion batteries.
Uses of synthetic graphite [edit]
Invention of a process to produce synthetic black lead [edit]
In 1893, Charles Street of Le Carbone discovered a process for making artificial graphite. In the mid-1890s, Edward Goodrich Acheson (1856–1931) accidentally made-up other style to produce synthetic plumbago after synthesizing carborundum (Si carbide Oregon SiC). He discovered that overheating carborundum, as opposed to pure carbon, produced almost pure graphite. While perusing the effects of high temperature on carborundum, he had found that silicon vaporizes at about 4,150 °C (7,500 °F), leaving the carbon hind end in graphitic carbon. This graphite became rich as a lubricant.[6]
Dean Gooderham Acheson's technique for producing atomic number 14 carbide and graphite is onymous the Dean Acheson mental process. In 1896, Dean Gooderham Acheson received a patent for his method of synthesizing graphite,[59] and in 1897 started commercial production.[6] The Acheson Graphite Co. was formed in 1899.
Synthetic graphite can as wel be prepared from polyimide and commerciallized.[60] [61]
Technological research [edit]
Highly oriented pyrolytic plumbago (HOPG) is the highest-timbre synthetic form of plumbago. IT is used in scientific research, particularly, as a length standard for scanner standardization of scanning poke into microscope.[62] [63]
Electrodes [edit]
Graphite electrodes carry the electricity that melts scrap branding iron and nerve, and sometimes address-cut iron (DRI), in electric arc furnaces, which are the vast majority of steel furnaces. They are made from crude oil coke subsequently it is mixed with coal tar pitch. They are then extruded and shaped, so baked to carbonize the binder (hawk), and last graphitized by heating it to temperatures approaching 3000 °C, at which the carbon atoms set into graphite. They can diverge in size upfield to 3.5 m (11 ft) long and 75 cm (30 in) in diameter. An increasing balance of international steel is made victimization electric spark furnaces, and the galvanic arc furnace itself is flattering more efficient, making more brand per tonne of electrode. An idea based on USGS data indicates that graphite electrode use was 197,000 tonnes in 2005.[46]
Electrolytic aluminium smelting likewise uses graphitic carbon electrodes. On a much smaller descale, synthetic graphite electrodes are used in electrical discharge machining (EDM), normally to make injection molds for plastics.[64]
Powderise and scrap [edit]
The powder is made by heating plant powdered petroleum Coke above the temperature of graphitization, sometimes with minor modifications. The graphite scrap comes from pieces of unusable electrode material (in the manufacturing stage or subsequently use) and lathe turnings, usually later crushing and sizing. To the highest degree synthetic graphite pulverization goes to carbon raising in steel (competitive with natural graphite), with some used in batteries and brake linings. According to the USGS, America agglutinative graphite powder and scrap production were 95,000 tonnes in 2001 (latest information).[46]
Neutron moderator [edit]
Special grades of synthetic substance graphite, such as Gilsocarbon,[65] [66] also find use atomic number 3 a matrix and neutron moderator inside nuclear reactors. Its under neutron crosswise also recommends IT for use in proposed fusion reactors. Care mustiness be taken that reactor-grade plumbago is unhampered neutron interesting materials such as boron, widely used as the seed electrode in dealing graphite deposition systems – this caused the failure of the Germans' World War II graphite-settled nuclear reactors. Since they could not isolate the trouble they were forced to consumption far more high-priced heavy water moderators. Graphite used for nuclear reactors is often referred to as cell organ graphite.
Other uses [edit]
Graphite (carbon) fiber and atomic number 6 nanotubes are also used in carbon fiber reinforced plastics, and in heat-resistant composites so much as strengthened carbon-carbon (RCC). Commercial structures made from carbon fiber graphite composites include sportfishing rods, golf gild shafts, wheel frames, sport car body panels, the fuselage of the Boeing 787 Dreamliner and pool cue sticks and have been with success employed in reinforced concrete. The mechanical properties of carbon character graphite-reinforced plastic composites and grey cast iron are strongly influenced by the role of graphite in these materials. In this linguistic context, the term "(100%) graphite" is often loosely used to refer to a pure mixture of carbon reinforcement and resin, while the term "asterid dicot family" is utilised for composite materials with additional ingredients.[67]
Modern smokeless powder is coated in graphite to prevent the buildup of static charge.
Graphite has been used in leastways three radar assimilatory materials. IT was mixed with rubber in Sumpf and Schornsteinfeger, which were used happening U-boat snorkels to reduce their microwave radar cross plane section. It was also used in tiles along early F-117 Nighthawk stealth smash fighters.
Graphite composites are used as absorber for piping-energy particles (e.g. in the LHC ray of light floor[68]).
Black lead rods when filed into shape are used as a tool in glassworking to pull wires hot molten glass.[69]
Black lead mining, beneficiation, and milling [edit out]

Graphite is well-mined by some open pit and tube methods. Graphite usually of necessity beneficiation. This may cost carried unfashionable aside hand-picking the pieces of gangue (rock) and reach-screening the product or by crushing the tilt and floating out the graphite. Beneficiation by flotation encounters the difficulty that graphite is very soft and "marks" (coats) the particles of gangue. This makes the "marked" gangue particles float off with the graphite, surrender impure decoct. In that location are two ways of obtaining a commercial concentrate or product: recurrent regrinding and afloat (up to sevener multiplication) to purify the centralise, or by acid leaching (dissolving) the gangue with hydrofluoric acid (for a silicate gangue) or hydrochloric acid (for a carbonate gangue).
In milling, the incoming graphite products and concentrates lav equal ground before being classified (sized or screened), with the coarser flake sizing fractions (under 8 meshwork, 8–20 mesh, 20–50 mesh) carefully salt-cured, and then the carbon copy table of contents are determined. Extraordinary standard blends can be prepared from the different fractions, each with a certain flake size distribution and carbon content. Custom blends can too be ready-made for separate customers who want a certain flake size distribution and carbon content. If flake size is superficial, the concentrate give notice be ground more freely. Typical end products include a fine powderise for use up as a slurry in oil drilling and coatings for foundry molds, carbon raiser in the blade industry (A posteriori graphite powder and powdered fossil oi coke can too represent used equally carbon raiser). Biological science impacts from plumbago Mills lie of air pollution including nongranular particulate exposure of workers and besides soil contamination from powder spillages leading to heavy metal contamination of soil.

According to the Federate States Geological Survey (USGS), world production of natural graphite in 2016 was 1,200,000 tonnes, of which the following major exporters are: China (780,000 t), Bharat (170,000 t), Brazil (80,000 t), Turkey (32,000 t) and North Korea (6,000 t).[70] Graphite is non yet mined in the United States. However, Westwater Resources is presently in the ontogeny stages of creating a pilot plant for their Coosa Black lead Mine near Sylacauga, Alabama.[71] U.S. production of inductive graphite in 2010 was 134,000 t valued at $1.07 billion.[46]
Occupational rubber [edit]
People can be unclothed to graphite in the workplace aside ventilation it in as substantially American Samoa through skin contact or eye contact.
United States [edit]
The Occupational Safe and Health Organisation (OSHA) has set the legal limit (permissible vulnerability restrain) for graphite photo in the workplace arsenic a time weighted average (TWA) of 15 million particles per cubic foot (1.5 mg/m3) over an 8-hour workday. The National Institute for Occupational Safety and Wellness (NIOSH) has set a recommended exposure specify (REL) of TWA 2.5 magnesium/m3 respirable dot over an 8-hour workday. At levels of 1250 mg/m3, graphite is immediately dangerous to life and wellness.[72]
Graphite recycling [edit]
The most common way of recycling graphite occurs when synthetic graphite electrodes are either manufactured and pieces are break off or lathe turnings are discarded for reuse, or the electrode (or other materials) are secondhand all the way down to the electrode bearer. A new electrode replaces the old one, but a sizable piece of the old electrode remains. This is crushed and sized, and the ensuant graphite powder is mostly old to raise the carbon content of molten steel. Graphite-containing refractories are sometimes also recycled, but often are non due to their low plumbago content: the largest-volume items, such as carbon-magnesite bricks that contain only 15–25% graphite, usually contain too little graphite to be worthwhile to recycle. However, some recycled carbon–magnesite brick is used equally the basis for furnace-recreate materials, and also crushed C–magnesite brick is used in scoria conditioners. While crucibles have a piercing graphite content, the loudness of crucibles used and then recycled is same small.
A high-quality flake black lead product that intimately resembles natural flake plumbago can Be made from steelmaking kish. Kish is a large-loudness neighbouring-liquid wild skimmed from the molten iron feed to a basic oxygen furnace and consists of a mix of black lead (precipitated out of the concentrated iron), caustic lime-rich slag, and some iron. The iron is recycled on-site, leaving a mixture of graphite and slag. The best recovery treat uses hydraulic classification (which utilizes a hang of irrigate to separate minerals by specific gravity: graphite is light and settles nearly last) to get a 70% graphite rough concentrate. Leaching this decoct with hydrochloric acid gives a 95% black lead product with a flake size of it ranging from 10 mesh down.
See also [edit]
- Acheson physical process
- Carbon character
- Nanotube
- Diamond
- Exfoliated graphite nano-platelets
- Fullerene
- Graphene
- Graphite embolism compound
- Graphitizing and not-graphitizing carbons
- Puffed
- Lonsdaleite
- Native element minerals
- Centre graphite
- Passive provok protection
- Graphite draftsmanship
- Pyrolytic atomic number 6
References [edit]
- ^ Liquid method: pure graphene production. Phys.org (Crataegus oxycantha 30, 2010).
- ^ Graphite. Mindat.org.
- ^ Graphite. Webmineral.com.
- ^ a b c Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Three-card monte C., explosive detection system. (1990). "Plumbago" (PDF). Handbook of Mineralogy. I (Elements, Sulfides, Sulfosalts). Chantilly, VA, US: Mineralogical Social club of USA. ISBN978-0962209703.
- ^ Sutphin, Saint David M.; James D. Bliss (August 1990). "Disseminated flake graphite and amorphous graphite deposit types; an analysis using grade and tunnage models". CIM Bulletin. 83 (940): 85–89.
- ^ a b c d e f graphite. Encyclopædia Britannica Online.
- ^ IUPAC, Compendium of Natural science Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "highly oriented shift graphite". doi:10.1351/goldbook.H02823
- ^ "Graphite". Minerals Database. Minerals Instruction Alliance. 2018. Retrieved 9 December 2018.
- ^ Maria, Lugaro (2005). Stardust From Meteorites: An Launching To Presolar Grains. Earthly concern Scientific. pp. 14, 154–157. ISBN9789814481373.
- ^ Hazen, R. M.; Downs, R. T.; Kah, L.; Sverjensky, D. (13 February 2013). "Carbon Stuff Evolution". Reviews in Mineralogy and Geochemistry. 75 (1): 79–107. Bibcode:2013RvMG...75...79H. Interior Department:10.2138/rmg.2013.75.4.
- ^ McCoy, T. J. (22 February 2010). "Mineralogical Evolution of Meteorites". Elements. 6 (1): 19–23. doi:10.2113/gselements.6.1.19.
- ^ Kucherov, O. P.; Rud, A.D. (2018). "Direct visual image of somebody molecules in building block crystals by electron cloud densitometry". Molecular Crystals and Liquid Crystals. 674 (1): 40–47. doi:10.1080/15421406.2019.1578510. S2CID 198335705.
- ^ Delhaes, Pierre (2000). "Polymorphism of C". In Delhaes, Capital of South Dakota (ed.). Graphite and precursors. Gordon & Breach. pp. 1–24. ISBN9789056992286.
- ^ Pierson, Hugh O. (2012). Handbook of carbon, plumbago, diamond, and fullerenes : properties, processing, and applications. Noyes Publications. pp. 40–41. ISBN9780815517399.
- ^ Delhaes, P. (2001). Graphite and Precursors. CRC Press. ISBN978-90-5699-228-6.
- ^ Chung, D. D. L. (2002). "Review Plumbago". Daybook of Materials Science. 37 (8): 1475–1489. DoI:10.1023/A:1014915307738. S2CID 189839788.
- ^ Pierson, Hugh O. (1993). Handbook of carbon paper, graphite, diamond, and fullerenes : properties, processing, and applications. Park Ridgepole, N.J.: Noyes Publications. ISBN0-8155-1739-4. OCLC 49708274.
- ^ Lipson, H.; Stokes, A. R. (1942). "A Newborn Structure of C". Nature. 149 (3777): 328. Bibcode:1942Natur.149Q.328L. doi:10.1038/149328a0. S2CID 36502694.
- ^ Latychevskaia, Tataiana; Son, Seok-Kyun; Yang, Yaping; Chancellor, Dale; Brown, Michael; Ozdemir, Servet; Madan, Ivan; Berruto, Gabriele; Carbone, Fabrizio; Mishchenko, Artem; Novoselov, Kostya (2019-08-17). "Stacking transition in symmetric graphite". Frontiers of Physical science. 14 (1): 13608. arXiv:1908.06284. Bibcode:2019FrPhy..1413608L. doi:10.1007/s11467-018-0867-y. S2CID 125322808.
- ^ IUPAC, Collection of Chemical Language, 2nd ed. (the "Gold Book") (1997). Online apochromatic reading: (2006–) "Symmetrical graphite". doi:10.1351/goldbook.R05385
- ^ a b Bundy, P.; Bassett, W. A.; Weathers, M. S.; Hemley, R. J.; Mao, H. K.; Goncharov, A. F. (1996). "The pressure-temperature phase and transformation diagram for carbon; updated through 1994". Carbon. 34 (2): 141–153. doi:10.1016/0008-6223(96)00170-4.
- ^ Wang, C. X.; Yang, G. W. (2012). "Physics and kinetic approaches of baseball field and concerned nanomaterials basket-shaped by laser ablation in liquid". In Yang, Guowei (ed.). Laser ablation in liquids : principles and applications in the readying of nanomaterials. Pan Stanford Pub. pp. 164–165. ISBN9789814241526.
- ^ Rock, Peter A. (1983). Chemical Thermodynamics. University Science Books. pp. 257–260. ISBN9781891389320.
- ^ Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. (2011). "The effects of firing conditions along the properties of electrophoretically deposited titanium dioxide films on graphite substrates". Diary of the European Instrumentation Society. 31 (15): 2877–2885. arXiv:1303.2757. doi:10.1016/j.jeurceramsoc.2011.07.007. S2CID 93406448.
- ^ Deprez, N.; McLachlan, D. S. (1988). "The analysis of the electrical conductivity of graphite conductivity of graphite powders during concretion". Journal of Natural philosophy D: Practical Physical science. 21 (1): 101–107. Bibcode:1988JPhD...21..101D. doi:10.1088/0022-3727/21/1/015.
- ^ Lavrakas, Vasilis (1957). "Textbook errors: Guest column. Cardinal: The lubricating properties of plumbago". Journal of Chemical Education. 34 (5): 240. Bibcode:1957JChEd..34..240L. Department of the Interior:10.1021/ed034p240.
- ^ Watanabe, N.; Hayakawa, H.; Yoshimoto, O.; Tojo, T. (2000). "The lubricating properties of graphite fluoride composites under both atmosphere and high vacuum condition". FY2000 Anchor – Based Research Announcement for Distance Utilization Research Report.
- ^ Yen, Bing; Schwickert, Birgit (2004). Origin of scurvy-friction behavior in plumbago investigated by surface x-ray diffraction, SLAC-PUB-10429 (PDF) (Report). Retrieved March 15, 2013.
- ^ Galvanic Corrosion Archived 2009-03-10 at the Wayback Machine. keytometals.com
- ^ "ASM Tech Notes – TN7-0506 – Galvanic Corrosion" (PDF). Atlas Specialisation Metals. Archived from the underived (PDF) on 2009-02-27.
- ^ Jones, Wrench (United States Air Force-Retired) Better Lubricants than Plumbago. graflex.org
- ^ "Weapons Lube in the Desert". September 16, 2005. Archived from the original on 2007-10-15. Retrieved 2009-06-06 .
- ^ "Good Engineering Practice/Corrosion". White lily Seven Club. 9 April 2003. Archived from the original on 16 September 2009.
- ^ Marshland, Harry; Reinoso, Francisco Rodríguez (2007). Activated carbon (1st male erecticle dysfunction.). Elsevier. pp. 497–498. ISBN9780080455969.
- ^ Boardman, John. "The Neolithic-Eneolithic Period" (PDF). The Cambridge ancient history, Volume 3, Part 1. pp. 31–32. ISBN978-0521224963. Archived from the original (PDF) happening 25 February 2013.
- ^ Norgate, Dean Martin and Norgate, Jean; Geography Section, Portsmouth University (2008). "Old Cumbria Gazetteer, black lead mine, Seathwaite". Retrieved 2008-05-19 . CS1 maint: fourfold names: authors list (link)
- ^ Wainwright, Alfred (2005). A Pictorial Guide to the Lakeland Fells, Western Fells. London: Frances Lincoln. ISBN978-0-7112-2460-5.
- ^ The Statutes at Large: From the ... Year of the Reign of ... to the ... Year of the Reign of . 1764. p. 415.
- ^ "History". Dixon Ticonderoga Party. Archived from the freehanded happening 7 April 2018.
- ^ a b Nguyen, Ahn (2003). Colloidal Scientific discipline of Floatation. p. 11. ISBN978-0824747824.
- ^ Cirkel, Fritz (1907). Graphite its Properties, Occurrence, Refinement and Uses. Ottawa: Canadian Department of Mines. p. throughout. Retrieved 6 April 2018.
- ^ Electro-Plating along Non-Bimetallic Substances. Spons' Shop Receipts. Vol. II: Dyeing to Japanning. Spon. 1921. p. 132.
- ^ Evans, John W. (1908). "V.— the Meanings and Synonyms of Graphite". Minutes of the Liberal arts Society. 26 (2): 133–179. Interior:10.1111/j.1467-968X.1908.tb00513.x.
- ^ Widenmann, Johann Friedrich Wilhelm (1794). Handbuch diethylstilboestrol oryktognostischen Theils der Mineralogie: Mit einer Farbentabelle und einer Kupfertafel. Crusius. p. 653.
- ^ Scheele, C. W. K. (1779). "Versuche mit Wasserbley; Molybdaena". Svenska Vetensk. Academ. Handlingar. 40: 238.
- ^ a b c d e f g h i j "Black lead Statistics and Information". USGS. Retrieved 2009-09-09 .
- ^ Almeida, Bruno Vidal First State; Neves, Elton Sylva; Sylva, Sidiney Nascimento; Vernilli Junior, Fernando (15 May 2017). "Attack Furnace Hearth Facing: Post Mortem Analysis". Materials Research. 20 (3): 814–818. doi:10.1590/1980-5373-mr-2016-0875.
- ^ Li, Yiwei; Li, Yawei; American ginseng, Shaobai; Chen, Xilai; Zhao, Lei; 51, Yuanbing; Li, Shujing (January 2014). "Prep of Ceramic-Bonded Carbon copy Block for Blast Furnace". Metallurgical and Materials Transactions A. 45 (1): 477–481. Bibcode:2014MMTA...45..477L. doi:10.1007/s11661-013-1976-4. S2CID 137571156.
- ^ Targray (August 27, 2020). "Graphite Anode Materials". Targray.
- ^ "How do centre diamond batteries work - prof simon Aug 26, 2020". YouTube. Archived from the new on 2021-10-30.
- ^ "Graphite/Metal Admixture Extends Material Life in High-Temperature Processes". Foundry Management & Applied science. 2004-06-04. Retrieved 2019-06-20 .
- ^ Harpist, Douglas. "graphite". Online Etymology Lexicon.
- ^ Ritter, Steve (October 15, 2001). "Pencils & Pencil Jumper lead". American Chemical Society.
- ^ "The History of the Pencil". University of Prairie State at Urbana–Champaign. Archived from the original on 2015-03-17. Retrieved 2013-02-15 .
- ^ "Electric Black lead Growing Demand From Electric Vehicles & Perambulating Electronics" (PDF). galaxycapitalcorp.com. July 20, 2011.
- ^ "Module 6: Media for 2-D Art" (PDF). Saylor.org. Retrieved 2 April 2012.
- ^ True semblance/appearance of the "Graphite, or Smokebox colors. List.nwhs.org. Retrieved on 2013-04-15.
- ^ Emery, Nicolas; Hérold, Claire; Marêché, Jean-François; Lagrange, Philippe (2008). "Deductive reasoning and superconducting properties of CaC6". Sci. Technol. Adv. Mater. 9 (4): 044102. Bibcode:2008STAdM...9d4102E. doi:10.1088/1468-6996/9/4/044102. PMC5099629. PMID 27878015.
- ^ Dean Gooderham Acheson, E. G. "Manufacture of Black lead", U.S. Patent 568,323, issued September 29, 1896.
- ^ Kato, Tomofumi; Yamada, Yasuhiro; Nishikawa, Yasushi; Ishikawa, Hiroki; Sato, Satoshi (2021-06-30). "Carbonization mechanisms of polyimide: Methodology to analyze carbon materials with nitrogen, oxygen, pentagons, and heptagons". Carbon. 178: 58–80. DoI:10.1016/j.carbon.2021.02.090. ISSN 0008-6223. S2CID 233539984.
- ^ Kato, Tomofumi; Yamada, Yasuhiro; Nishikawa, Yasushi; Otomo, Toshiya; Sato, Hayato; Sato, Satoshi (2021-07-12). "Origins of peaks of graphitic and pyrrolic nitrogen in N1s X-ray photoelectron spectra of carbon materials: quaternary nitrogen, tertiary aminoalkane, operating room thirdhand aminoalkane?". Journal of Materials Scientific discipline. 56 (28): 15798–15811. Bibcode:2021JMatS..5615798K. doi:10.1007/s10853-021-06283-5. ISSN 1573-4803. S2CID 235793266.
- ^ R. V. Lapshin (1998). "Automatic lateral standardization of tunneling microscope scanners" (PDF). Review of Technological Instruments. 69 (9): 3268–3276. Bibcode:1998RScI...69.3268L. doi:10.1063/1.1149091. ISSN 0034-6748.
- ^ R. V. Lapshin (2019). "Drift-insensitive distributed calibration of probe microscope scanner in nanometer range: Real mode". Applied Surface Science. 470: 1122–1129. arXiv:1501.06679. Bibcode:2019ApSS..470.1122L. Interior Department:10.1016/j.apsusc.2018.10.149. ISSN 0169-4332. S2CID 119275633.
- ^ Hugh O. Pierson – Enchiridion of Carbon, Black lead, Diamonds, and Fullerenes: Processing, Properties, and Applications – Alfred Noyes Publication ISBN 0-8155-1339-9
- ^ Arregui-Mena, J. D.; Bodel, W.; et al. (2016). "Attribute variability in the mechanical properties of Gilsocarbon". Carbon. 110: 497–517. doi:10.1016/j.carbon.2016.09.051.
- ^ Arregui Mena, J.D.; et atomic number 13. (2018). "Characterisation of the spatial variability of material properties of Gilsocarbon and NBG-18 victimization random fields". Diary of Nuclear Materials. 511: 91–108. Bibcode:2018JNuM..511...91A. doi:10.1016/j.jnucmat.2018.09.008. S2CID 105291655.
- ^ Cooper, Jeff. What is the best corporate for a tennis racquet?. lawn tennis.some.com
- ^ Yurkewicz, Katie. "Protecting the LHC from itself" (PDF). Symmetry Magazine.
- ^ Olmec Advanced Materials (2019). "How black lead is used in the glass and fibreglass industries". Retrieved 19 January 2019.
- ^ "Mineral Commodity Summaries 2020" (PDF). Federal Minerals Information Center. USGS.
- ^ Jeremy Practice of law (2018-05-16). "Westwater Resources acquires A Plumbago". Retrieved 2020-02-22 .
- ^ "CDC – NIOSH Pocket Guide to Chemic Hazards – Graphite (natural)". www.cdc.gov . Retrieved 2015-11-03 .
Far reading [blue-pencil]
- C.Michael Hogan; Marc Papineau; et al. (December 18, 1989). Stage I Environmental Site Assessment, Asbury Graphite Mill, 2426–2500 Kirkham Street, Oakland, California, Earth Prosody report 10292.001 (Composition).
- Felix Klein, Cornelis; Cornelius S. Hurlbut, Jn. (1985). Manual of Mineralogy: after Dana (20th male erecticle dysfunction.). ISBN978-0-471-80580-9.
- Taylor, Harold A. (2000). Graphite. Business Multiplication Executive Commodity Reports. London: Mining Journal Books ltd. ISBN978-1-84083-332-4.
- Taylor, Harold A. (2005). Graphite. Industrial Minerals and Rocks (7th ed.). Littleton, CO: AIME-Society of Mining Engineers. ISBN978-0-87335-233-8.
External golf links [edit]
| | Wikimedia Commons has media accompanying Black lead. |
- Battery Grade Plumbago
- Plumbago at Minerals.net
- Mineral galleries
- Inorganic & Exploration – Map of World Graphite Mines and Producers 2012
- Mindat w/ locations
- giant valency structures
- The Graphite Page
- Video lecture on the properties of graphite by Prof. M. Heggie, University of Sussex
- CDC – NIOSH Pocket Guide to Chemical Hazards
Where to Buy Plastic Tip Plugs for Graphite Shafts
Source: https://en.wikipedia.org/wiki/Graphite
0 Response to "Where to Buy Plastic Tip Plugs for Graphite Shafts"
Post a Comment